In-stent restenosis
Physiological background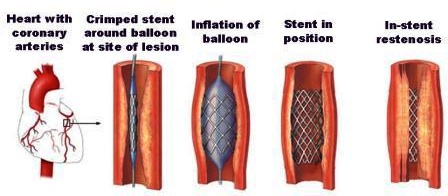
A stenosis is an abnormal narrowing in a blood vessel. Interventional techniques to treat stenotic arteries involve deployment of a metallic tubular mesh (stent) into the vessel walls. As a maladaptive response of the artery to the injury caused by the stent deployment activated vascular healing processes lead to abnormal tissue growth. Intense inflammatory response causes neointimal hyperplasia followed by smooth muscle cell proliferation and migration.
Complex automata
A Complex Automaton (CxA) for a multi-scale multi-science problem can be described by:
- A collection of Single scale models based on cellular automata, lattice Boltzmann models and Agent Based models,
- A Scale Separation Map (SSM) where the sub-scale systems occupy well-defined regions in regard to their spatial and temporal scales,
- Coupling Templates: the way those sub-processes interact with each other.
Model
A Multiscale Simulation Coupling Library and Environment (MUSCLE), developed in COAST, is used to set up and run simulations as a CxA. In a CxA simulation of ISR using a simplified Connection Scheme, three single scale models (BF, SMC, DD, and one init kernel) interact with each other using smart conduits. Smart conduits convert data to match representations in the receiving model. Some common conduit functionalities (interpolation, scaling etc.) exist as templates and can be combined in these conduits.
Stent deployment (INIT)
The stent deployment results in an injury involving the removal of endothelial cells and the rupture of internal elastic lamina due to stretching of vessel tissue. In the INIT agent of the CxA, these features are dynamically simulated to match a certain Gunn injury score. Resulting cell configurations serve to initialize the SMC model.
Smooth muscle cells (SMC)
SMC hyperplasia is the most prominent aspect of ISR. In the current model of SMC tissue, SMCs are simulated as individual agents with mechanical interaction and internal dynamics.
Migration of the SMCs is governed by mechanical interactions based on cell-cell adhesion, mechanical cell-cell repulsion, and friction with the tissue.
SMC proliferation is regulated by the Cell Cycle which has three stages:
- G0: quiescent state
- G1: first growth stage
- G2: final stage leading to Mitosis
Progression through the cell cycle is controlled by a set of biological rules depending on the presence of neighbouring cells, wall shear stress due to the friction of blood flow with exposed surface cells and drug concentration.
Blood flow (BF)
Using the lattice-Boltzmann method, haemodynamics is simulated numerically on a spatial grid fine enough to resolve the flow lines near to stent strut.
- flow simulation is run to equilibration of static/periodic flow field
- wall shear stress and OSI (oscill. shear index) distributions are passed to SMC.
- if tissue growth changes voxelized vessel geometry, BF is re-run.
Drug diffusion (DD)
Drug Eluting Stents, which are coated with anti-proliferative drugs, have proven to play an Important role in preventing / inhibiting the tissue re-growth. The process of drug elution is modelled using a generic anisotropic diffusion equation, solved numerically by Cellular Automata – Finite Difference Scheme. Drug was released from stent strut (source) at constant rate into the SMC (tissue) and the lumen (sink).
Preliminary Results
Preliminary results show that stent design parameters such as diameter of strut and the strut shape can lead to different restenosis rates within 14 days post deployment. 2D and 3D models will be developed/tunedfurther in order to cover in-vivo data.
Acknowledgements
- The MeDDiCA project, which is a Marie Curie Initial Training Network (MC ITN) funded by the European Union under EU-FP7. www.meddica.eu
- The COAST Project. www.complex-automata.org
Bibliography
- The MUSCLE library: http://muscle.berlios.de .
- Hoekstra A.G., Lorenz E., Falcone J.-L., Chopard B., Towards a Complex Automata Framework for Multi-scale Modeling, Int. J. Mult. Comp. Eng., vol 5, 491-502 (2007).
- Evans D., Lawford P., Gunn J., Walker D., Hose R., Smallwood R., Chopard B., Krafczyk M., Bernsdorf J., Hoekstra A.G., The Application of Multiscale Modelling to the Process of Development and Prevention of Stenosis in a Stented Coronary Artery, Phil. Trans. Roy. Soc. A, 366, 3343-3360.
- A. Caiazzo, D. Evans, J.-L. Falcone, J. Hegewald, E. Lorenz, B. Stahl, D. Wang, J. Bernsdorf, B. Chopard, J. Gunn, R. Hose, M. Krafczyk, P. Lawford, R. Smallwood, D. Walker, and A. Hoekstra, A Complex Automata approach for In-stent Restenosis: two-dimensional multiscale modeling and simulations, J. of Computational Sci, accepted for publication, 2010.



